Can You Measure the ABV of Rum with a Refractometer?

After reading this Reddit post showing the viability of measuring ABV of distilled spirits with a refractometer, I was interested in confirming this with my own refractometer, and seeing how well it works for rum specifically. But let’s back up.
Why would you want to measure the ABV of a spirit? Doesn’t it say that on the bottle?
A totally fair question to which I have 3 responses:
- I’m a nerd and it’s fun.
- ABV labels in the US can legally be off by 0.3% and can be off by more due to proofing mistakes.
- If you assume the the ABV label is correct (or close enough) you can use measured ABV to estimate the quantity of additives—coloring and sugar—in a rum. More on this in a follow-up post.
Hydrometers vs Refractometers
Now that we’ve spelled out why we might want to measure the ABV of a rum, let’s get a little background on refractometers out of the way:
Hydrometers
Hydrometers measure the density of a liquid using buoyancy. It’s a bit of an oversimplification, but if your liquid is very dense, a hydrometer won’t sink into it very much, and if your liquid isn’t very dense, a hydrometer will sink a lot. You can measure how far the hydrometer sinks, and this tells you how dense your liquid is.
Hydrometers are often used in brewing, winemaking, and distillation because the density of a liquid can help you understand how much sugar, alcohol and water are in a liquid. But a hydrometer doesn’t directly measure sugar or alcohol; it measures density. Generally speaking, you must have some preexisting data (like the initial amount of sugar you added into a fermentation wash) in order to go from density to something like alcohol content.
A side note: generally people using hydrometers refer to “specific gravity” instead of density; specific gravity is the ratio of a material’s density to the density of water. Up until a few weeks ago, I would’ve told you the density of water was 1g/mL, but apparently, 0.999974g/mL is the number we’re using these days. The ratio of any number and 1 is just the original number (and the ratio of any number and something very close to 1 is very close to the original number), so these numbers end up being essentially the same. The main difference is that density has units (e.g. g/mL) and specific gravity is unit-less, so it’s a little easier to work with in different unit systems. I generally stick to density.
Refractometers
Refractometers measure the refractive index of a liquid. If you stick a pencil into a glass of water, the pencil will appear to bend. Essentially, the refractive index of a liquid is how much it bends light (this is another oversimplification). A refractometer works by shining a light through a liquid, and measuring how much that light is bent (refracted) by the liquid.
Because different mixtures had different refractive indexes, you can use a refractometer to estimate the concentration of things. But similar to the hydrometer, it isn’t directly measuring the concentration of anything—it’s measuring the refractive index of a liquid, then you need some data to compare that to in order to calculate something like dissolved sugars or ABV.
Practical Differences
We’ve unpacked some of the physical differences between hydrometers and refractometers, but there are also some practical differences:
- There are digital and analog versions of both, but digital refractometers are more common and cheaper than digital hydrometers
- Refractometers often require a much smaller sample for every measurement taken
- Hydrometers seem to be more commonly used in establishing ABV in spirits, so more data exist for them
Differences 1 and 2 are, in my opinion, clear advantages of refractometers. Digital refractometers are less prone to user error (are you supposed to measure at the top or the bottom of the meniscus?), can be easily calibrated, and can do automatic temperature control. Some hydrometer setups can require a 200mL sample to get a reading, but refractometers can make do with 1-2mL. Unless you’re drinking the sample afterward, a refractometer test is much less wasteful.
But then there’s difference 3. Remember neither of these devices measures ABV directly, they measure some physical property that, with a set of reference data and some assumptions, you can use to estimate ABV. In large part, this post is an effort to remedy this by adding a set of reference data for deriving the ABV of distilled spirits—specifically rum—with a refractometer.
Methodology
Onto the nerdy stuff:
- Dilute a neutral high-proof spirit (Everclear 95) to various ABVs, and record the ºbrix reading on my Milwaukee Refractometer
- Build a model of the relationship between ABV and ºbrix
- Test if this model built on data from a neutral spirit can effectively measure the ABV of rums that are known to have no additives
This third step is important because even though they’re not considered “additives” there are tons of compounds in rum not found in a neutral spirit (i.e. vodka). These compounds, mostly originating from the fermentation and aging processes can affect the reading of a refractometer (or hydrometer for that matter). If the effect is too great, then this would limit the viability of measuring the ABV of rum with a refractometer.

Results
Here’s the table of readings I got for step 1. Note that I attempted to measure at regular intervals, but I don’t have the steadiest hand, and so I just recorded the actual ABV of the samples I prepared (ultimately it doesn’t change the math).
And here’s that data as a scatter plot:
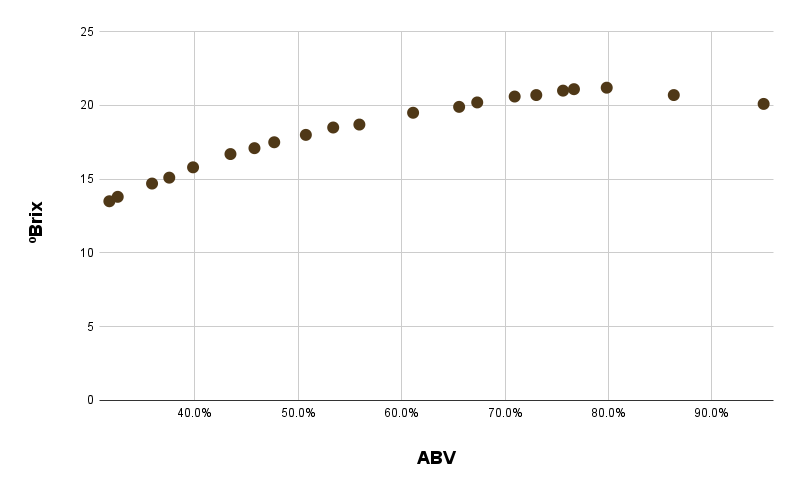
Conveniently, the graph matches that original reddit post, and looks like a relatively smooth trend. It’s clearly not linear, but fitting to anything above a cubic function would probably result in overfitting. We can also see that because the graph starts going down after ~80% ABV, there’s really no way of knowing if a high reading corresponds to a little bit above 80%, or a little bit below 80%.
The resulting cubic I got was ABV = 0.0572*brix^3 - 2.4305*brix^2 + 37.9142*brix - 178.0087. For the less mathematically inclined, this is just a formula we can use to predict ABV based on a refractometer reading. But the real question is how well it predicts ABV. If our predictions are constantly wrong, the formula isn’t very useful.
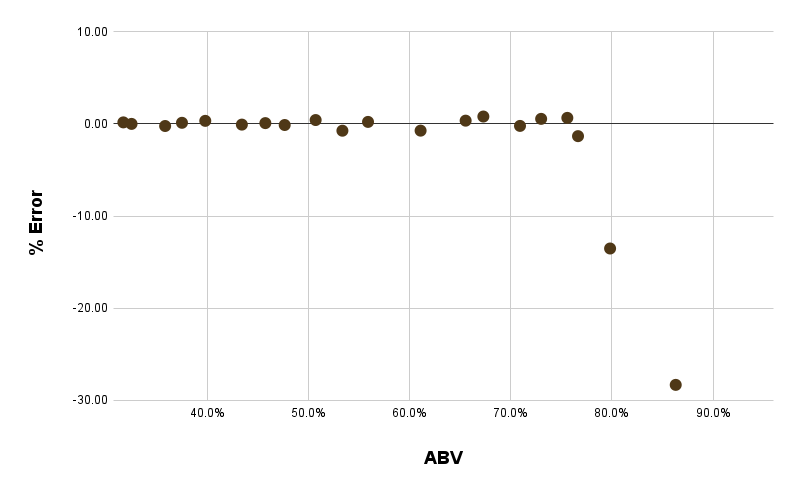
Luckily, the formula is pretty accurate up until we hit that peak at 80%. For actual ethanol solutions between 30% and 76% ABV, the refractometer method always predicts within 1% of the actual value. At 80% ABV, the error is up to 1.3%, and by 95% ABV (the full-strength neutral spirit) the error leaps to almost 30%.
So tentatively, I would conclude this data suggests you can effectively use a refractometer to measure the ABV of neutral spirits under 80% ABV. Thankfully, the highest ABV regularly seen in rum is 75.5 (i.e. 151 proof), which is about as high as I’d feel comfortable measuring.
But so far this is just data from a neutral spirit. If we want to use this method for rum, we need to see how accurately it performs on rum, and if the impacts of fermentation and aging change things enough for the method to be useless.
For this test, I gathered a diverse set of rum that hopefully cover most of the bases: molasses and cane juice based, proofs from 40% to 63% ABV, ages from 0 to 12, from 100% column still to 100% pot still. And importantly, all of them have preexisting test results showing they are free of any additives. Here are the rums I tested, their ABVs, their refractometer reading, and the error achieved by trying to predict their ABV through two different methods (discussed below):
Everclear Model Error is simply how wrong the prediction of the original model we built above—which on average was 2.8% away from the true value—not terrible but not great. Everclear + Rum Model Error is based on a new model where I included the ABV and refractometer data of the rums themselves. This model performed significantly better, averaging a little under 1% error, meaning on average we’d expect this method to get us within 1% of the true ABV of a rum with no additives.
As a final check, I tested this model on a few holdout rums. Essentially, because we included the rum data in our model, it makes the above check sort of like an open-book test (i.e. the model has seen the right answers). Testing it on rums that weren’t in the training data let us get a sense of how well it will generalize to rums it hasn’t seen before. Here I tested Doorly’s 12, Foursquare’s ECS 2009, and Clement’s Blanc Rhum Agricole. Together their mean absolute error was .48%, and the highest error was the Clement, with a 0.7% error.
Conclusion
A consumer-grade refractometer seems to be an imperfect but useful tool for measuring the ABV of rum without additives to within a ~1% range. While it wouldn’t satisfy the TTB, which requires readings to be accurate to within 0.3% of the actual ABV, it may be useful for many enthusiast and hobbyist applications, and (depending on the sensitivity of the refractometer to sugar presence) may give a reliable signal for substantial quantities of additives.
Baie des Tresors Flowers of the Winds
Neat RatingMixer RatingOverall Rating:
HSE VSOP Martinique Rhum Agricole
Neat RatingMixer RatingOverall Rating:
Baie des Tresors Fruit of the Rains
Neat RatingMixer RatingOverall Rating:



Residual sugers and additives in the rum can throw off the mesurmaents as you note, you would optimaly have both refrectometer and density readings from a hydrometer or similar to get more accurat results
alcohol by vol = 1.646 * b – 2.703 * (145 – 145 / s) – 1.794
Where:
b = °Brix reading (from refractometer)
s = specific gravity (from hydrometer)
https://www.vinolab.hr/calculator/alcohol-from-hydrometer-refractometer-en27